Working principle
The three primary components of a Li-ion battery cell are the negative and positive electrodes and the electrolyte. Li-ion batteries rely on the shuttle of Li-ions back and forth between the two electrodes during charge-discharge cycles. The basics of Li-ion batteries are explained using by adopting the most common battery chemistry, i.e. the combination of a graphite anode and a LiCoO2 cathode. Strictly speaking, the roles of anode and cathode switch when going from discharging to charging state. Here we use the terms defined during discharging as referred to in the battery literature. During charging, the electrolyte carries positively charged lithium ions from the cathode to the anode.
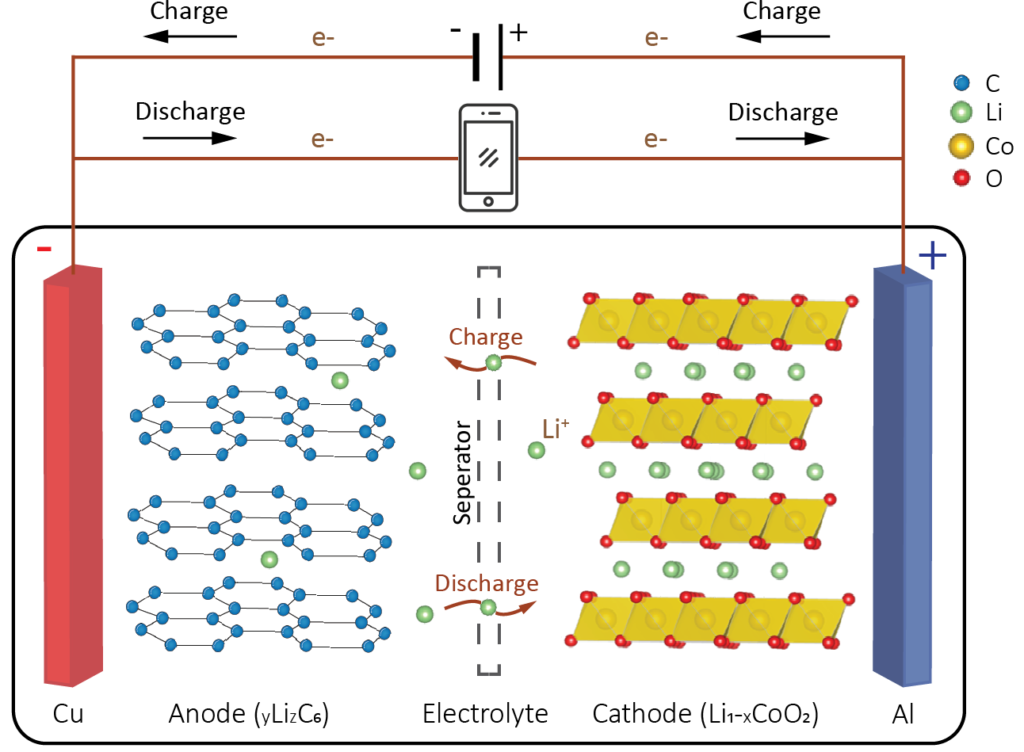
Conventionally, Li-ion batteries use liquid electrolytes, containing a high concentration of a Li salt (e.g., LiPF6 or LiClO4) to ensure ionic conductivity between the two electrodes. The electrolyte does not conduct electrons. Moreover, the separator prevents physical contact between the anode and cathode and blocks the flow of electrons inside the battery. The release of lithium ions from the cathode induces free electrons which lead to the generation of an electrical current flowing through the positive current collector and the external circuit towards the negative current collector and into the anode. While the battery is discharging, the opposite reaction happens. The anode releases lithium ions, which diffuse towards the cathode. The latter generates a flow of electrons through the negative current collector and the external circuit towards the positive current collector and into the cathode, providing an electric current to the device being powered (cell phone, computer, etc.).
The working mechanism of a Li-ion battery can be understood more clearly when considering the reactions occurring at each electrode. During charging, the cobalt is partially oxidized from Co3+ into Co4+ and Li-ions (Li+) are released:
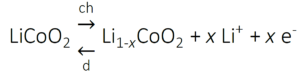
The reverse occurs during discharging: electrons are accepted by the cathode, Co4+ is reduced to Co3+ and intercalation of Li-ions takes place to balance the charge. To prevent irreversible structure changes of the LiCoO2 electrode, not all Li+ ions can be removed and therefore, x should be less than 0.5. Upon charging, Li+ ions are transported into a graphite (C6) electrode and are subsequently reduced:
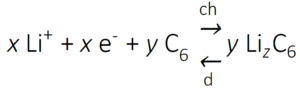
Here, z = x/y and has a maximum value of 1. During discharging the reverse occurs, where the graphite releases electrons and de-intercalates Li-ions which dissolve into the electrolyte. The full reaction taking place (left to right: discharging, right to left: charging) is then:
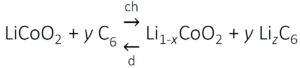
The reactions at both anode and cathode must be highly reversible for hundreds of charge/discharge cycles.
When the LiCoO2 is fully delithiated, the battery is fully charged. The output potential of the cell finds its origin in the electrochemical potential difference of the electrodes. In general, the standard redox potential of the electrode redox reaction should be low for the anode (e.g., 0.065 – 0.25 V vs Li/Li+ for graphite) and high for the cathode (e.g., 3.9 V vs Li/Li+ for LiCoO2), leading to a high cell voltage. In the lithium battery community, the electrode potentials are referred to the Li/Li+ system since in a lithium battery the anode typically is Li metal or lithium intercalated in graphite with a redox potential very close to the metal Li/Li+ electrode.